A New Regime: India’s Approach to New Drugs and Vaccines
Context:
In recent developments, the Drug Controller General of India has granted clearance, under the provisions of ’emergency use authorization,’ to a new mRNA vaccine for COVID-19. Developed by Pune-based Gennova Biopharmaceuticals, this vaccine demonstrates efficacy against the dominant Omicron variant.
- The approval of GEMCOVAC-OM holds significance as it showcases India’s capability to produce mRNA vaccines, known for their potential in rapid production and scalability, which could be valuable in combating future viruses.
Relevance:
GS-02 (Government Policies and Interventions in various sectors)
Prelims:
- Types of Vaccines
- Virus Strain and mutation
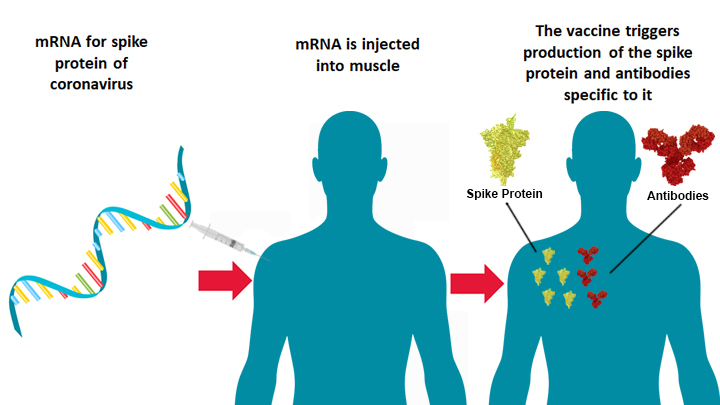
- Spike Protein
Mains Questions:
- Evaluate the challenges associated with the emergency use authorization regime for drug and vaccine approvals and propose measures to ensure safety and monitor adverse reactions. (150 words)
Dimensions of the article:
- Progressive Testing and Emergency Use Authorizations
- The Need for a Streamlined Regulatory Process
- Balancing Flexibility and Safety
Progressive Testing and Emergency Use Authorizations:
- Traditionally, vaccines undergo a series of testing stages, progressing from laboratory experiments to animal trials and finally expanding human test cohorts. These stages ensure the safety and efficacy of the vaccine, disqualifying those that cause harm or fail to outperform existing alternatives.
- However, the COVID-19 crisis necessitated a departure from this risk-averse approach, prompting global drug regulators to allow vaccine manufacturers to combine multiple stages while evaluating efficacy. This flexibility, inherent in the framework of ’emergency use authorizations (EUA),’ enabled the expedited evaluation of experimental formulations.
- While the U.S. Food and Drug Administration (FDA) has a long-standing history of evaluating novel drug and vaccine candidates, India’s regulatory system has primarily focused on assessing formulations already approved abroad. This distinction highlights the historic challenges of mistrust, arbitrary decrees, and lax regulations that have plagued clinical trials in India.
The Need for a Streamlined Regulatory Process:
- Although the New Drugs and Clinical Trials Rules of 2019 facilitated EUA for COVID-19 vaccines in India, the country’s credible regime of phased clinical trials and independent regulation for new drugs is still in its nascent stages.
- It is essential for India to establish a streamlined regulatory process that eliminates non-essential steps, accelerates approvals, and remains vigilant in monitoring the safety and adverse reactions associated with new drugs and vaccines.
Balancing Flexibility and Safety:
- While maintaining the ability to expedite critical interventions, India must prioritize safety and ensure a robust system for monitoring adverse reactions from new drugs and vaccines. This necessitates striking a delicate balance between the need for accelerated approvals and thorough safety evaluations.
- By adopting a hawk-eyed approach toward potential adverse reactions, India can instill confidence in the public and prevent any unwarranted harm caused by hasty regulatory decisions. Developing a comprehensive mechanism for post-authorization surveillance and addressing any emerging safety concerns should be integral to the regulatory framework.
Way Forward:
To enhance India’s approach to new drugs and vaccines, several measures can be implemented.
- Firstly, there should be a concerted effort to establish an independent and robust regulatory authority that can conduct thorough assessments of novel formulations. This includes building the capacity to evaluate the safety and efficacy of emerging technologies such as mRNA vaccines.
- Secondly, enhancing public trust in clinical trials requires transparent and evidence-based decision-making processes. Stringent regulations must be implemented to ensure ethical conduct, minimize arbitrary decrees, and eradicate any historical flaws that have plagued India’s clinical trial landscape.
- Lastly, continuous monitoring and surveillance systems must be established to proactively identify and address any adverse reactions or safety concerns associated with newly approved drugs and vaccines.
Conclusion:
India’s recent authorization of Gennova Biopharmaceuticals’ mRNA vaccine marks a significant milestone in the country’s vaccine landscape. While the emergency use authorization regime provided the flexibility needed during the COVID-19 crisis, it is crucial for India to develop a streamlined regulatory process that prioritizes safety and vigilance against adverse reactions. By focusing on a balanced approach that combines accelerated approvals with robust monitoring systems, India can foster public trust, advance healthcare innovation, and effectively address future health challenges.