Central Drugs Standard Control Organization
#GS-02 Healthcare, Governance
For Prelims
Central Drugs Standard Control Organisation (CDSCO):
- CDSCO works under Directorate General of Health Services, Ministry of Health & Family Welfare.
- It is the National Regulatory Authority (NRA) of India.
- The headquarters of CDSCO is in New Delhi and there are six functioning central drug testing laboratories under CDSCO.
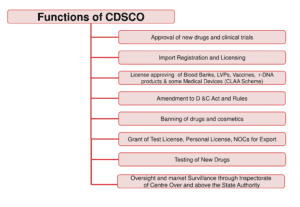
Functions of CDSCO:
CDSCO is granted a multitude of responsibilities under Drugs and Cosmetics Act, these include;
- Giving approval of New Drugs,
- Conducting Clinical Trials,
- laying down the standards for Drugs,
- maintain control over the quality of imported Drugs in the country and
- coordinating the activities of State Drug Control Organizations by providing expert advice with a view to bring about the uniformity in the enforcement of the Drugs and Cosmetics Act.
- CDSCO along with state regulators, is given the joint responsibility for granting licenses of certain specialized categories of critical Drugs.
- They include blood and blood products, I. V. Fluids, Vaccine and Serum.
Drug Controller General of India
- The Drug Controller General of India (DCGI) is the head of CDSCO.
Functions of DCGI:
- The DCGI establishes the standards for the manufacturing, import, sales, and distribution of drugs in India.
- DCGI also responsible for regulation of medical and pharmaceutical devices.
- The DCGI is the appellate authority in case of disputes with respect to the quality of the drug.
- The DCGI is responsible for preparing and maintaining the national reference standard for drugs.
- They ensure the uniformity in the implementation of the Drugs and Cosmetics Act.
- They are responsible for the training of Drug Analysts of the State Drug Control Laboratories and other Institutions.
- They are also in charge of the analysis of cosmetics received from the CDSCO as survey samples.
- The DCGI is also the central licensing authority for medical devices which fall under the Medical Device Rules 2017.




